Three Reasons Why We Care about Design for Manufacturability
Some might say that process design isn’t fun or exciting, but, for us at ClearPath Medical, designing for a product’s manufacturability is what gets our gears spinning.
For me personally, I’ve spent most of my life thinking about Design for Manufacturability (DFM). As early as the age of 7, having studied under my Father’s hand at his mold and die shop, I learned the importance of DFM. The hum of machinery, the precise cuts of steel and the high level of precision and foresight required to build a superior product has always fascinated me. Simple assumptions within a design could result in poor yields (product/component waste), or affect the overall functionality and look to the end-user.
What is DFM?
DFM is the process of designing products with the goal of eliminating potentials for error.
It also ensures that a design can be manufactured cost-effectively and with the right materials for the desired environment and use case. DFM is a critical step in new product development and sets the stage for considerations in the overall tool design and process planning.
When executed well, DFM will assure both quality and productive parts with low waste.
Here are three reasons why we put DFM at the forefront of our product design decisions.
1 – It ensures we can actually manufacture products
DFM starts with one of the most important aspects of a new medical cable design project- collecting all of the design inputs.
Design inputs are the electrical, mechanical and environmental requirements of your product that will be used as a basis for device design and product qualification. In addition to electrical, mechanical and environmental requirements, it’s important to understand how your product will be used.
After finalizing design inputs into design drawings (which are also used for the basis of tool design), basic 3D (or machined) Prototypes can be made to qualify the design concept before full tooling production. It enables us to ‘simulate’ what it would be like to build the product in a production environment at our facility.
In manufacturing, it’s sometimes very challenging to achieve the desired features of a part or performance of a system on the first try. Prototyped parts allow us to quickly pivot if needed so that we can go back to the drawing board to make modifications before production tooling is built.
2 – DFM impacts the aesthetics, feel and functionality of the finished product
DFM includes all designs for bill of materials (BOM) items and final assembly, such as mold layouts/tooling design and manufacturing process design.
Only with correctly designed tools is it possible to achieve the correct look, tactility, accuracy, and functionality of your product. Design requirements might include:
- Encapsulation: An inner-mold for the sealing of critical components (such as PCBs, resistors, capacitors)
- Flexibility: For added flexibility, strain reliefs maintain the structural integrity, flexibility and overall robustness between the cable material and connector or grommet
- Gripping: Non-slip finger grips built into the connector over-mold for ease of use
- Security: Latching features which lock connectors in place and prohibits disconnection during use
- Handling: Uniquely designed handles for maneuvering flexibility, often desirable for surgical applications.
As an aesthetics example, sections of molds may leave parting lines and tool marks on a product. Parting lines are the border-lines in which draft angles change direction – it is the dividing line that splits the tooling halves of a molded part. The location of these parting lines may impact the product’s look and feel.
As part of the process, these types of decisions are made and approved by both the ClearPath Medical design team and our customer.
3 – It drives the overall timeline
We’ve seen cases where prior manufacturers of products ignore DFM altogether. This almost always results in frustrated, unhappy customers who expected to receive their products weeks ago.
When creating a project timeline, allotting time towards good DFM practices should always be included. Depending upon the quality requirements and complexity of the medical device environment, it generally may take several weeks and iterations between our teams to land on a solid manufacturing path.
It’s necessary to have every detail of medical cable assembly projects verified and confirmed with both our design team and our customer’s team during frequent communications and design reviews. Throughout the development process, ClearPath Medical will participate in design reviews in compliance with design control requirements.
During design reviews, we share CAD 3D models, 3D printed samples or machined prototypes for approval before beginning tool fabrication.
This establishes a certain confidence level for ‘freezing’ the design in order to start production steps. It helps eliminate potential mistakes that may take a lot of time and money to recover from at a later stage. While it seems that making time for adjustments is contradictory and may slow the overall project, DFM best-practice is the tortoise that wins the race in medical device manufacturing.
DFM Behind the Scenes: Poka-Yoke
As our engineers analyze and assess new designs for manufacturability, they provide feedback to our customers on how particular elements of an assembly may be modified to enable mistake-proofing or poka-yoke mechanisms. The purpose of a poka-yoke mechanism is to eliminate product defects by preventing human errors as they occur. One example of mistake-proofing is to intentionally offset otherwise symmetric features so they cannot be placed or assembled incorrectly.
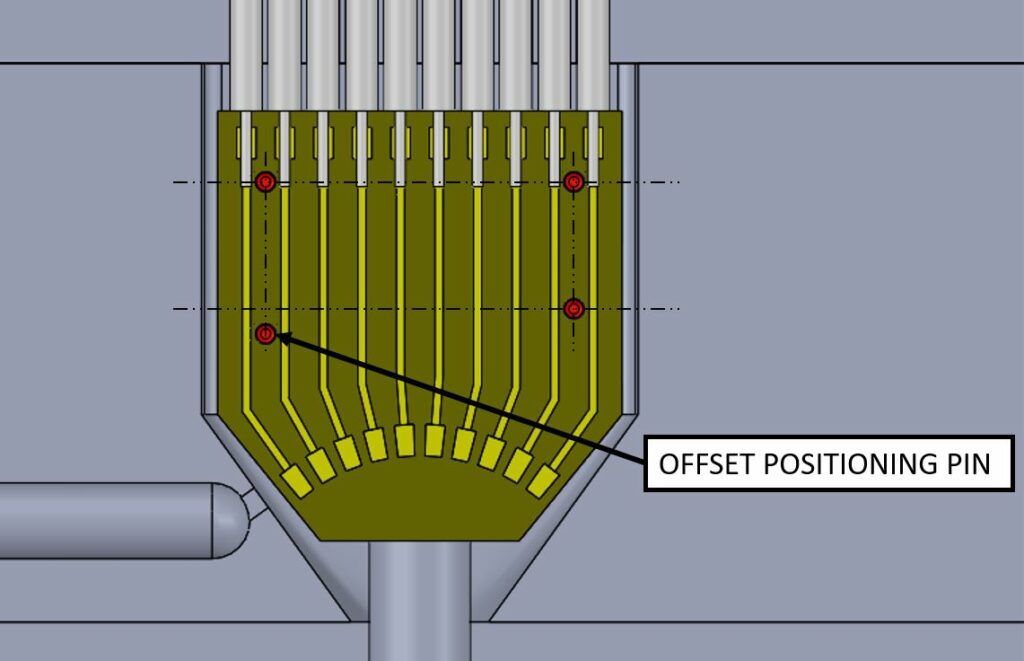
Some poka-yoke methods are invisible to the final product, such as the example above. Others may impact the aesthetics of the final product and should be considered early in the design process. In both cases, mistake proofing is an essential characteristic of DFM that allows us to maintain optimal manufacturing yields and drive efficiencies in our production process, ultimately benefiting our customers and the end-user.
Ready to Implement Design for Manufacturability for Your Next Project?
Have you taken DFM into consideration of your product’s design? Or, do you need assistance in DFM? We’re here to help.
If you’re curious about the markets we serve, download our latest Solution Brief.
Thanks for reading!

