CLEANING, DISINFECTION AND STERILIZATION DESIGN CONSIDERATIONS FOR MEDICAL CABLES
In the wake of the novel Coronavirus (COVID-19), we know that we have an important part to play in ensuring the safety of either those within the healthcare supply chain, and those on the forefront in delivering care to those infected and their families. In light of recent events, we’ve had some customers inquire about any new or special cleaning instructions required for this virus. This month we wanted to share our knowledge on general cleaning, disinfection and sterilization of medical devices, as well as what we know about the impact of Coronavirus on medical assemblies today. We want to emphasize that the Center for Disease Control (CDC) changes the list of effective disinfectants frequently – if there are any questions, contact us or follow the latest Environmental Protection Agency (EPA) list.
USAGE – SINGLE OR MULTI PATIENT USE
Unless designed for single use, medical devices and cable assemblies will almost certainly require cleaning, disinfection and possibly sterilization. Depending upon the intended use, cleaning may range from a wipe down with soap up to the elimination of all microorganisms by sterilization.
The recommended method to clean medical cables and connectors, and equally important; how they are likely to be cleaned should be addressed early in the design process so that the most appropriate materials and manufacturing processes will be used.
KNOW THE RISK-BASED CLASSIFICATION
Based on the risk of contamination spreading from a device to a patient, three levels of disinfection are defined based on a strategy developed by Dr. Earle Spaulding in the 1960’s. Dr. Spaulding was the Chair of the Department of Microbiology and Immunology at Temple University School of Medicine between 1949 and 1972.
When considering disinfection or sterilization, both Spaulding and the EPA classify medical cables and medical lead-wires as “non-critical”. Most medical cable assemblies encounter contact with unbroken skin and do not normally come in contact with mucous membranes. Non-critical devices typically require only cleaning and low-level disinfection. If cables or wires come in contact with broken skin or mucous membranes, a higher level disinfection is required.
CABLE JACKET MATERIAL SPECIFICATION
We dedicated an entire blog to this subject since we think it’s one of the most critical components that helps create effective and consistent cleaning. The jacket material is not only the part of the medical cable assembly that is most visible; it plays a large role in the performance of the overall finished medical device. A cable jacket offers mechanical, chemical and environmental protection to the conductors and components within the jacket.

Because the cable jacket is exposed, the conditions the cable will be used under and how it will be cleaned or disinfected should be considered early in the design stage.
The following tables offer guidelines as to the suitability materials based on various cleaning, disinfection and sterilization methods. These materials are commonly used for cable jackets and molded assemblies.
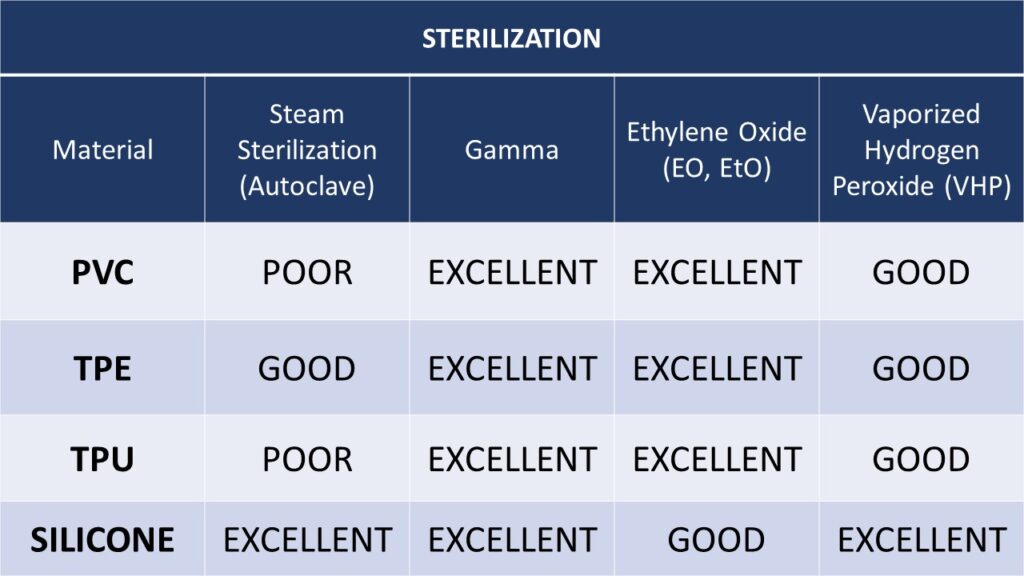
SELECTING THE RIGHT CABLE JACKET AND OVER-MOLD MATERIALS FOR CLEANING
- PVC is one of the lower cost materials used in cable assemblies. It is commonly used to insulate conductors, as cable jacket material and also for molded components. PVC offers good resistance to alcohols, most solvents and alkali, but is not suitable for steam sterilization by autoclave.
- TPE/TPR (Thermoplastic Elastomer or Thermoplastic Rubber) – These materials are often referred to by the trade name of Santoprene®. TPE/TPR materials have excellent chemical resistance and are suitable for cleaning and disinfection by most methods. With suitable design and manufacturing processes, TPE/TPE cable assemblies can withstand up to fifty or more steam autoclave cycles.
- TPU – Thermoplastic Polyurethane offers excellent mechanical properties including abrasion resistance and tear strength. Disadvantages of polyurethane material include poor resistance to some common cleaning agents and high temperatures making them unsuitable for steam sterilization by autoclave.
- Silicone – Silicone is both very flexible and offers very high flex life. It is the most common choice where a high number of steam sterilization cycles are required. Silicone cables can also be disinfected with most common solutions. However, silicone is less durable than other materials used for wire and cable jackets. It is easily cut and offers lower tear resistance materials.
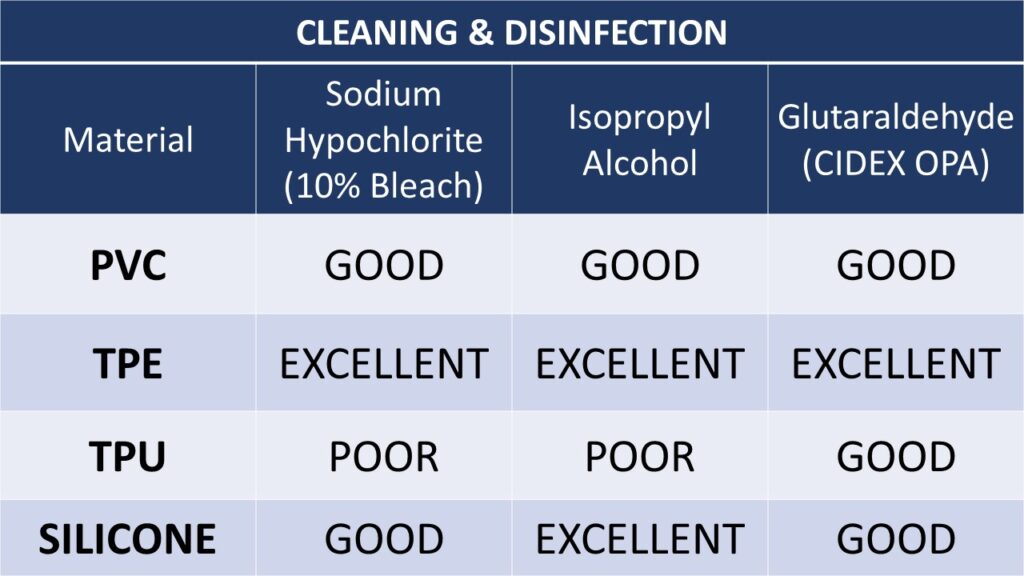
CLEANING AND DISINFECTION ISSUES
When used at higher than the recommended concentrations, cleaning and disinfection agents can damage medical cables and leads. An all too common error is that disinfection solutions such as glutaraldehyde or sodium hypochlorite (bleach) are used at much higher concentrations than recommended, causing damage to medical devices such as cables and leads.

Due to the nature of healthcare facilities, medical devices and cable assemblies are often cleaned with wipes saturated with Quaternary Ammonium compounds containing didecyl dimethyl ammonium chloride. While these wipes are very efficient for cleaning and disinfecting, they are specifically designed to be used on hard, non-porous surfaces such as stainless steel counters and tables. With continued use these cleaning agents will degrade many plastics, including cable and wire jackets, leading to reduced service life. Common trade names of wipes designed to disinfect hard surfaces are “CaviWipes” and “Sani-Cloth.”
KEEPING UP WITH THE LATEST DISINFECTION GUIDELINES
Several of the chemicals that have been qualified for medical device disinfection clear all viruses in the Corona family. We are aware of specific solutions that include 70% minimum IPA and 10% bleach solution. Cidex OPA, for example, has been tested against coronavirus and demonstrated to be efficacious. Since EPA’s list of registered disinfectants changes frequently, we suggest all our customers to use the EPA’s guidance when a question arises. If you have specific questions related to COVID-19, we suggest this link: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. The advice from the CDC is for hospitals and caregivers to follow the device IFU for cleaning and sterilization practices.
In summary, cleaning, disinfection and sterilization can significantly affect the service life of a medical cable assembly and we’re here to help guide our customers and prospects in selecting the appropriate materials for these efforts. How the product will be cleaned and disinfected should be considered at an early stage of product development so that the most appropriate materials are selected.
A special thank you to the scientific, healthcare and supply chain community for working tirelessly to ensure the health and safety of those impacted by COVID-19.

