THE FUNDAMENTALS OF CABLE ASSEMBLY OVERMOLDING
In this blog, we will explore the processes and tooling behind successful medical cable assembly injection overmolding.
Critical medical devices are often used in very harsh environments. These ‘harsh’ environments include frequent chemical wipe-downs, contact with bodily fluids and even steam-sterilization cycles. That’s why injection overmolding is one of the key factors in medical devices performing successfully over the duration of the expected life cycle. Not only do overmolded features encapsulate and provide flex/bend protection to electrical connections, they ultimately improve a product’s mechanical performance. Additionally, overmolding offers the aesthetic appeal to a medical cable assembly that can align with a company’s product and brand strategy.
2-STEP SUCCESSFUL OVERMOLD PROCESS:
First- Pre-molding
Prior to any type of molding, it’s likely that there’s exposed materials that are sensitive and prone to quick mechanical break-down if not covered, like exposed conductors or printed circuit boards. In this case, a pre-mold may be required. A pre-mold (also may be known as the inner mold) acts behind-the-scenes to augment mechanical integrity and encapsulate terminations along with some portion of a multi-conductor cable or leadwire jacket. In this case, a pre-mold will act as a rigid structure that resists the strain caused by the bending and twisting of the cable or leadwire when in use by a clinician. The best materials for pre-molds are Polypropylene or Polyethylene polymers. However, there may be some scenarios where some components cannot withstand the direct pressures and temperatures of injection overmolding. In these cases, we would recommend low-pressure molding or pre-molded housings/clamshells which can be utilized as the inner component in place of a pre-mold.


Second– Overmolding
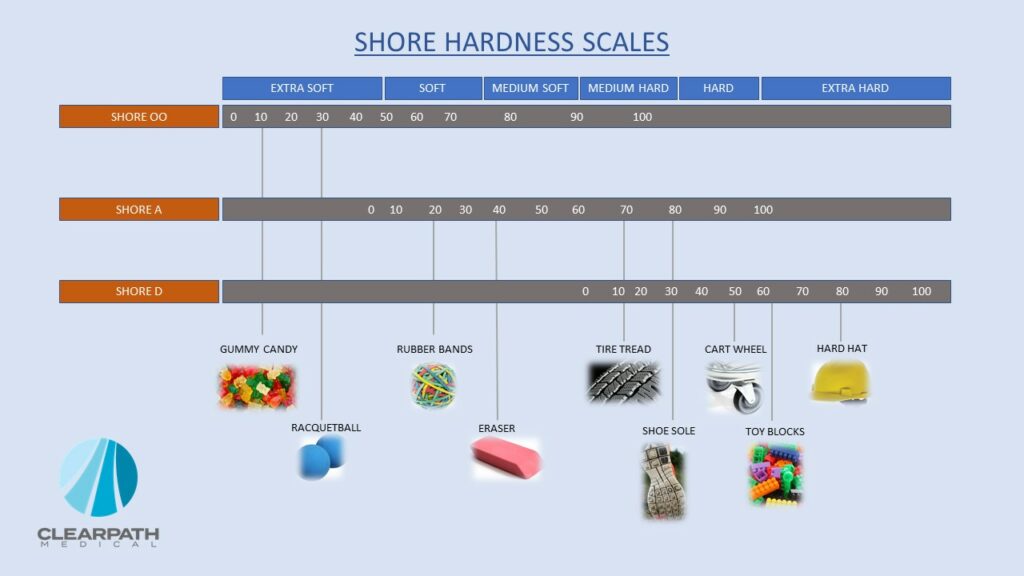
Above, we talked about the pre-mold which essentially provides mechanical integrity and the protection that medical cable assemblies require. However, it does not yield a very aesthetically pleasing end-result, and oftentimes doesn’t allow for medical device Original Equipment Manufacturers (OEMs) to include their visual directions and branding. Overmolding provides that aesthetic and tactile pleasing product that also adds additional protection against the environment in which the cable assembly is being used. Several options exist in the selection of suitable thermoplastic materials for overmolded assemblies. Flexible elastomers (rubber-like polymers) are used to aid in bend reliefs and yoke transitions. We find that elastomers with a Shore A 80 durometer or less works best. Durometer shores are essentially the ‘hardness’ of that polymer, and Shore A can range from very soft and flexible to hard and limited flexibility. The selection of hardness will be dependent upon flexibility requirements, aesthetics, and the environment in which the medical device will be used. On the other hand, we recommend that harder elastomers (Shore A 90 Durometer or greater) are used when some degree of rigidity is required. In these case, segmented strain reliefs could be coupled with harder durometer elastomers for medical devices that require rigidity in some portions and flexibility in others.
OVERMOLDING IN ACTION
Typically, single component parts are molded in horizontal clamping injection molding machines where pelletized polymers are heated and injected under high pressure into a mold. Medical cable assembly overmolding works similarly, but instead uses vertical clamping machines that inject molten material into the parting line of the mold. This allows operators to easily place and remove finished assemblies from the machine.
Often, medical cable assembly OEMs have several different configurations of their finished medical cable assembly based on the number of leads, cable diameters, jacket colors, etc. This is important information to capture during the product specification phase, as mold tooling may need to be designed for interchangeability that can accommodate the various configurations. This may mean that each mold component for injection overmolding may be unique within the same product line. This may include the cavities, loading and gripper bars, and strain relief tools.
Lastly, it is crucial to consider the following for high-quality, long-lasting medical device assemblies that are overmolded:
Overmold Tool Design:
- Selection and understanding of how certain substrates and cable jacket densities will perform is also a key characteristic when designing overmold tooling
- Wall thickness, shot size, and runner/gate design should be designed provide a robust manufacturing process
- Location and size of gates should be discussed with OEM
Overmold Tool Materials:
- Aluminum, stainless steel and hardened steel are the most common for overmolded medical cable assemblies
- The choice of overmold tooling material should be driven by production volumes and product life expectancy
SUMMARY
Both the process and tooling of overmolding is an essential part of custom medical cable assembly projects. At ClearPath Medical, we make it a fundamental focus of our OEM’s project from concept through design freeze. Are your medical device assemblies performing well within their life expectancy and environments, or do you think your team could use an improvement to your overmold solution? If so, contact us at ClearPath Medical. We are ready to help! Be sure to also follow us on LinkedIn for company updates, news, and more!

