THE RECIPE FOR PRODUCT SPECIFICATIONS
Back in 2019 we wrote a blog called better ingredients = better cables, which emphasized the importance of taking ten specific steps to ensure that finished medical assemblies not only meet customer expectations, but have the opportunity to exceed expectations. One of those critical steps is the establishment of product specifications and from our point of view, skipping this step is like forgetting to add flour to your cake. If a solid set of product specifications are not established, a gap is left between what is assumed to be known and what is understood, ultimately affecting the ability to deliver highly qualified medical cable assemblies with fewer revisions. That is why, after all the design inputs have been gathered, the drafting of product specifications is a critical step in the design and development of a new medical cable assembly, connector, or associated device – what we like to call, the recipe. The complexity of the product being developed dictates the level of detail required to be included in the product specification. Let us explore what goes into a well-thought-out medical device product specification:
1 – UNDERSTAND THE PRODUCT’S PURPOSE
It is helpful for a design team to understand what need the proposed medical device is intended to fulfill, who will be using the device and how the product is expected to work in its environment. As an example, the design of a medical cable assembly would likely be different if the intended user is a patient in their home environment verses clinician in a healthcare office. From there a description of the medical device (product) can be written and may include statements regarding the environment in which the product will be used.
Most important is that a product description should assist the team tasked with designing the product so they can understand the nature of the product and what its intended use is.
2 – CAPTURE THE DESIRED LOOK
It is helpful to the design team to understand what the finished medical device is expected to look like and overall aesthetics. The appearance of a medical cable assembly will often be one of the key factors in determining how the user will perceive the product. Some examples of descriptive statements that can help facilitate the design are:
- Smooth edges and no sharp corners
- Reduce features that can trap dirt and make cleaning difficult
- Rigidity or pliability, hardness or softness of user interface surfaces: e.g. “soft rubbery feel”
- A specific color (possibly to match corporate branding) or the desired effect of a color: e.g. “to reduce visible marks from normal handling”
- Surface finish: e.g. “dull to eliminate glare or reflections”
If these ideas are not documented and made available to the design team, the product may not turn out as expected.
3 – TAKE INTO ACCOUNT THE PHYSICAL AND DIMENSIONAL CHARACTERISTICS
Once the general concept of the new medical device is established, physical and dimensional characteristics can be captured. Once the cable assembly elements are considered, the actual physical and dimensional characteristics shall be documented. A few examples include size constraints, length of finished cable assembly, raw cable diameter, cleaning requirements and more.
4 – CAPTURE THE ELECTRICAL & MECHANICAL PARAMETERS
Because medical cables assemblies typically carry a signal, power or both, electrical requirements are a significant part of the device specification. It is important to take into consideration items such as number of conductors, cable and wire shielding, maximum voltage and current capacity, resistance, and many other electrical components.
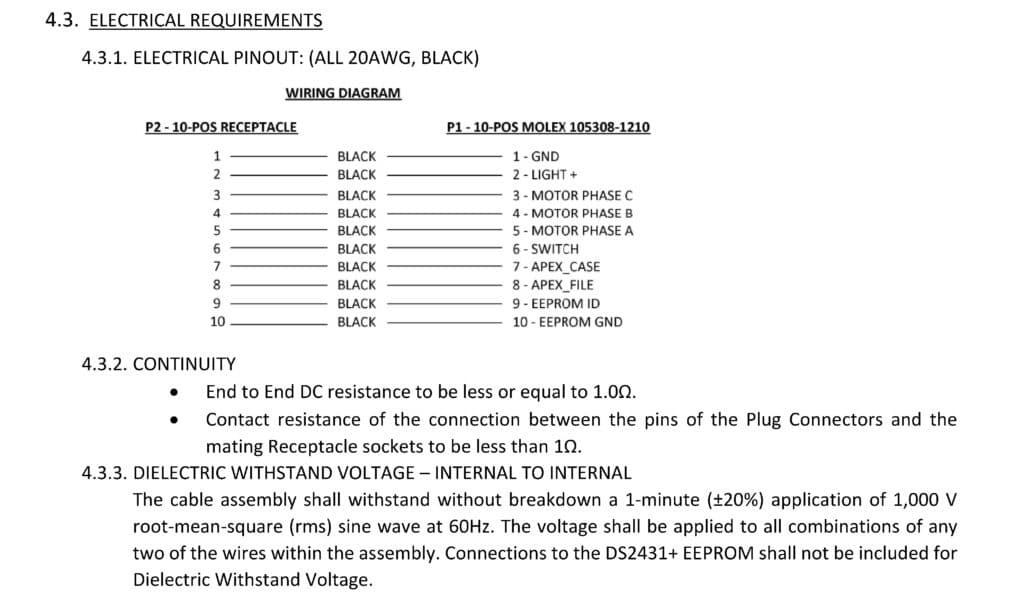
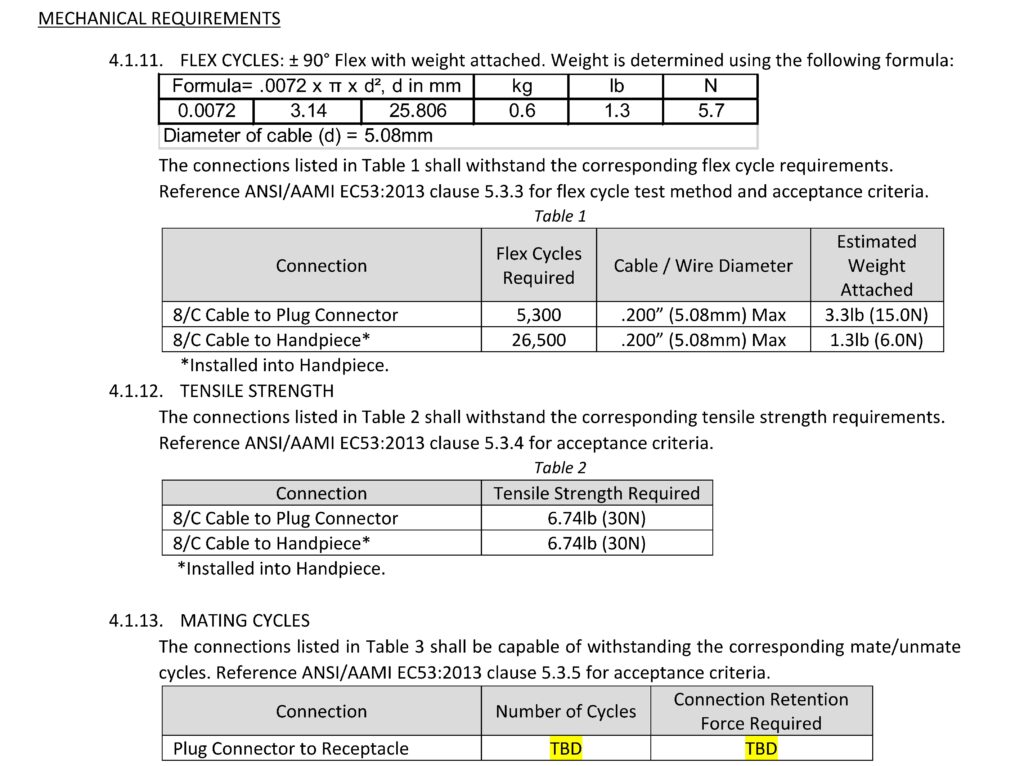
Along with electrical requirements, defining mechanical requirements for a cable assembly or connector is necessary before design work can begin. Some examples include: flex life for each cable or wire termination point, tensile strength for each cable or wire termination, number of mate/un-mate cycles, connector retention force and many more mechanical requirements.
5 – EVALUATE REGULATORY REQUIREMENTS
We would be remiss if we didn’t mention everyone’s favorite topic – regulatory. It is critical that regulatory requirements be evaluated and documented within the product specification. This includes items such as FDA, Medical Device Directive, IEC, ANSI/AAMI EC53, ISO 10993 (Biocompatibility) RoHS, REACH and UL/CSA.
6 – CONSIDER HOW PRODUCTS WILL BE LABELED AND PACKAGED
Along the lines of regulatory standards, medical cables require labeling and marking for identification in order to comply with these standards. Products should not be released without proper labeling and packaging.
Defining and documenting labeling, marking and packaging requirements as part of the original product specification will help ensure an on-time product release. Some examples include, but not limited to brand labeling, regulatory requirements labeling, UDI (Unique Device Identifier) part/lot/date coding, and more.
7 – INCLUDE CLEANING AND STERILIZATION REQUIREMENTS
Unless a medical device is going to be designed for single use, it will need to be cleaned. Even so, some medical cable assemblies designed for single use may require sterilization prior to being used. Regardless of single or multi-use, cleaning may range from a simple wipe down with soap to the elimination of all micro-organisms by sterilization. Cleaning and disinfection requirements must be considered in the early product design so that appropriate materials and production processes will be specified. Common cleaning, disinfection and sterilization methods include:
- Green soap or alcohol-free hand soap
- 2% Glutaraldehyde
- Sodium hypochlorite (bleach) solution
- Isopropyl Alcohol
- Quaternary Ammonium Compounds
- Autoclave
- Ethylene oxide
- Gamma radiation
- Sterrad (gas plasma)

In order for the finished medical device to successfully survive cleaning, disinfection and sterilization, these requirements must be included in the product specification in order for the design team to address them when materials and components are selected.
8 – NOTE THE DESIRED PRODUCT LIFECYCLE
Consider what the expectations for product reliability are and how long the medical device is expected to last under normal use? It is nearly impossible to control how a product is used once it is manufactured. Because of this, the reliability of a product should be defined in specifications that can be verified by testing.
SUMMARY
The list above is not exhaustive – depending on product and customer asks, there are many other things that can be included in a Product Specification. At ClearPath Medical, we feel that capturing the full gamut of a medical device’s requirements is important, starting with the items we outlined above. And in preparing a detailed product specification and obtaining approval by all relevant parties at the start of a project can oftentimes shorten the overall project timeline. Equally important, a well-prepared product specification will often reduce the overall cost by reducing or eliminating expensive mid-project design changes. Contact us to learn more!

