Choosing the Right Medical Connectors
Read time: 4 minutes
When it comes to medical cable assembly design and development, choosing the right medical connector is an important piece of the puzzle. In some cases, the challenge is knowing what specifications to look out for- but it doesn’t have to be!
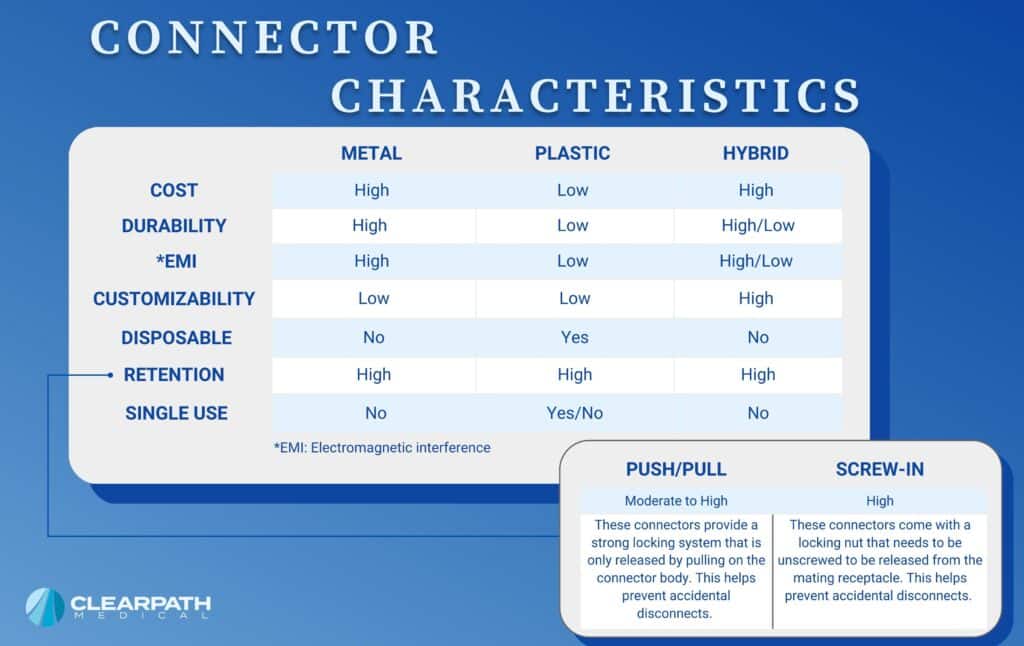
Medical connectors are utilized differently depending on their intended purposes. Connectors have various functions, specific/unique materials, and different degrees of durability. Determining a medical connector’s requirements affects the integrity and performance of a medical cable.
This month’s blog provides a simple outline of connector types, connector components, design qualifications, and possible considerations for connectors used in medical cable assemblies.
Types of Medical Connectors
There are expectations for medical connectors to withstand and endure different environments when being built in the medical manufacturing industry. For example, therapeutic, surgical, and diagnostic environments utilize reusable cable assemblies for applications such as vital signs monitoring, defibrillation equipment and more; however, there are situations where a disposable connector is more beneficial than a reusable connector.
In any case, a good starting point is the basic understanding of types of medical connectors.

Here’s a list of the common types of connectors:
Push-Pull Connectors
- Strong locking & latching systems that are only released by pulling on the connector body. This helps prevent accidental disconnects.
- Low/High Cost Options
- High connector retention
Screw-In Connectors
- Locking nut that needs to be unscrewed to be released from the mating receptacle. This helps prevent accidental disconnects.
- Higher Cost
- High connector retention
Disposable Connectors
- Replaced after each use
- Inexpensive/low cost
- Used for single use assemblies (sanitation reasons)
- Low durability
Metal Shell
- High cost
- High durability
- High resistance to electromagnetic interference
- High resistance to harsh environments
Plastic Shell
- Lower cost
- Lower durability
- Low resistance to harsh environments
Hybrid Connectors
- Moderate/High cost
- Very custom designs
- Incorporated more than one type of media for signals (e.g., electrical, pneumatic, optics, fluidic)
Components of a Connector
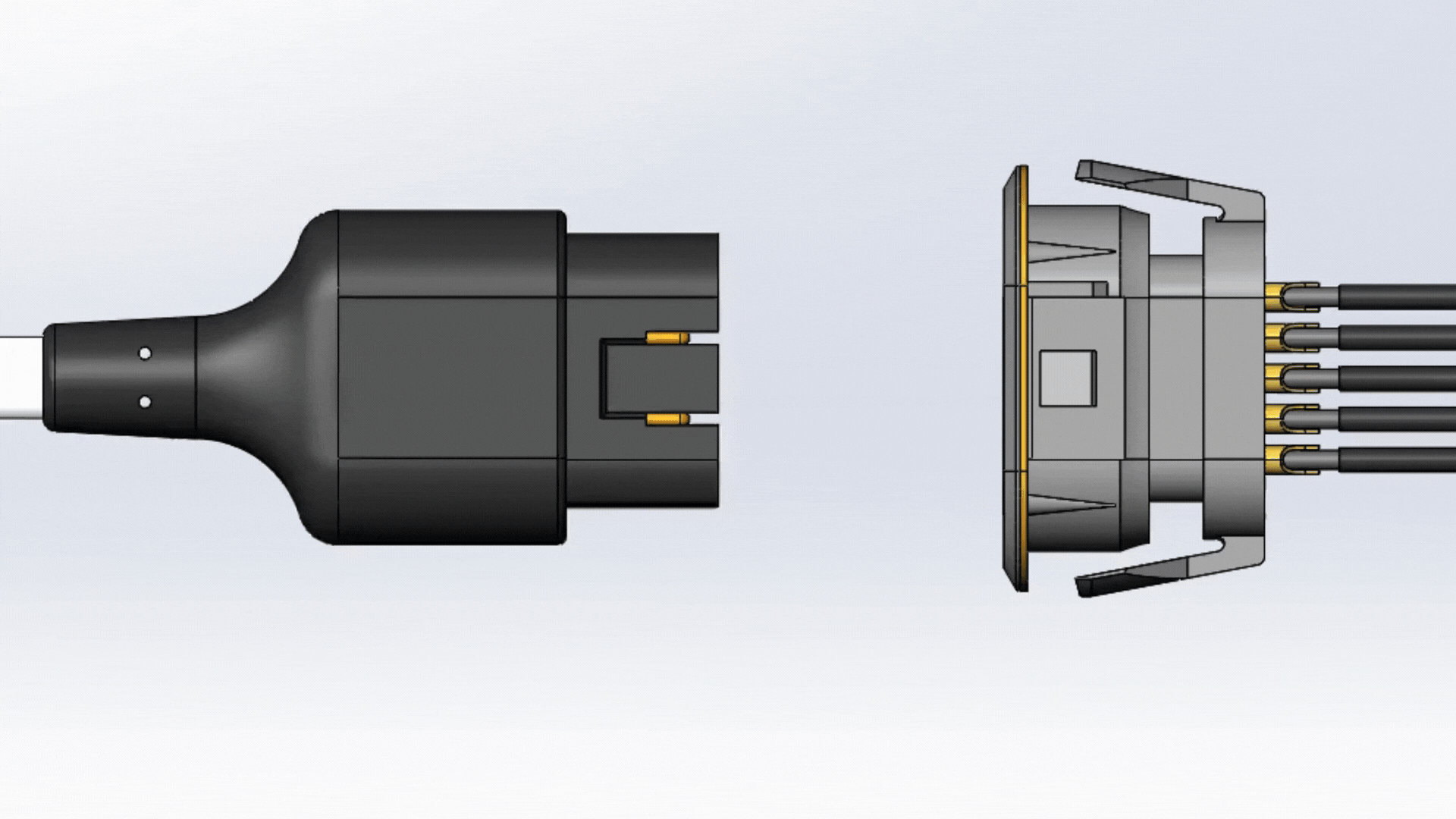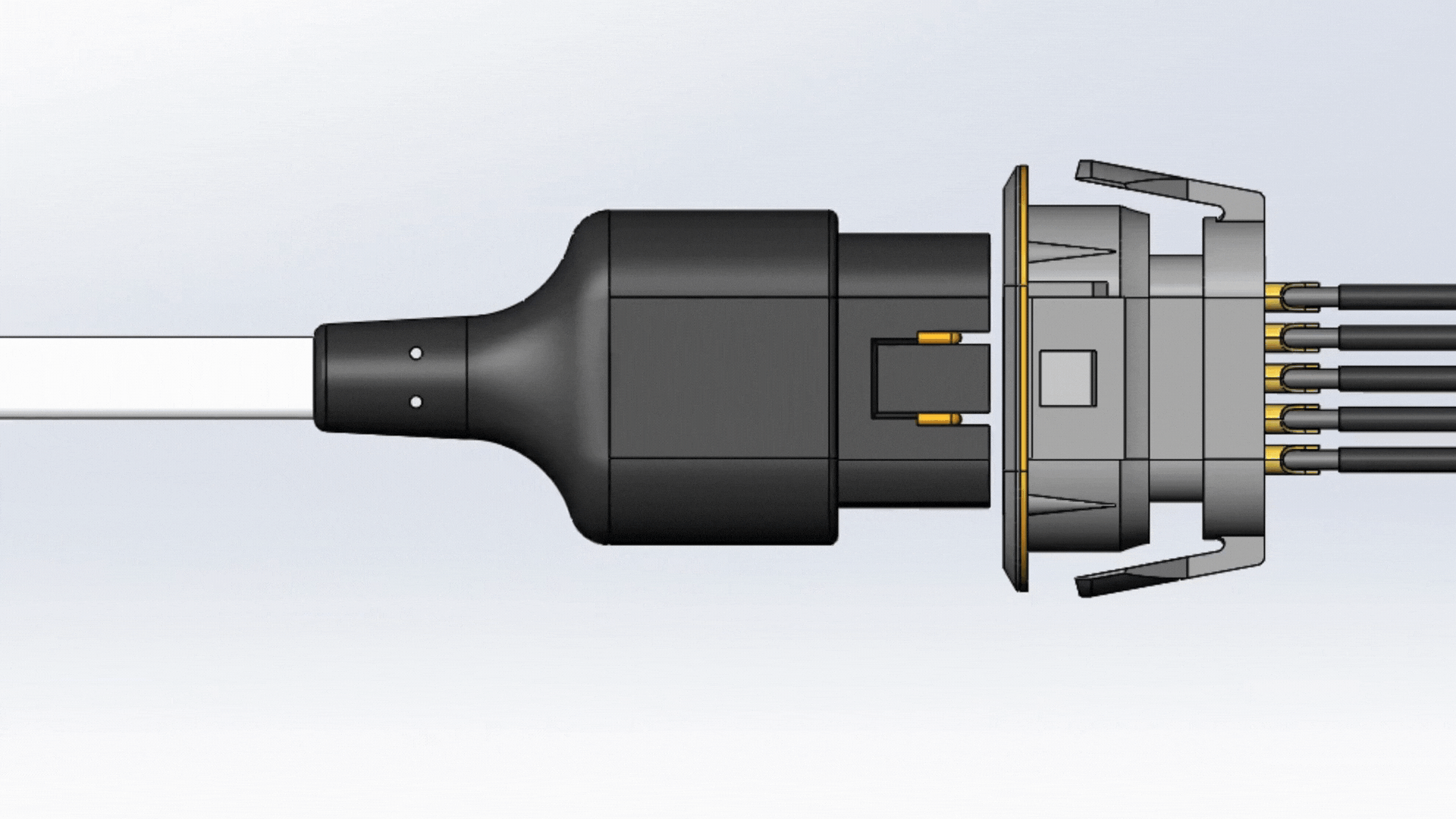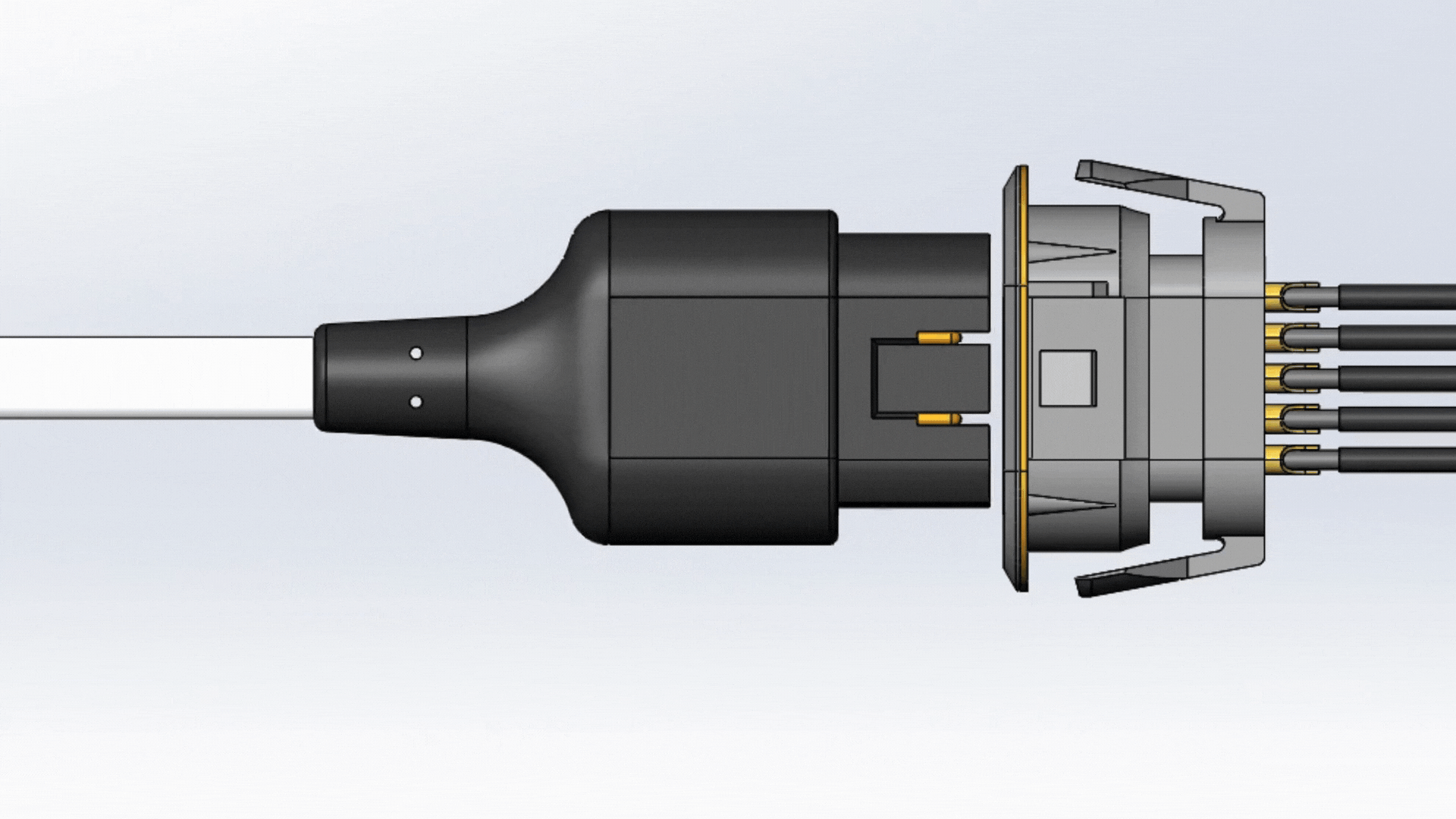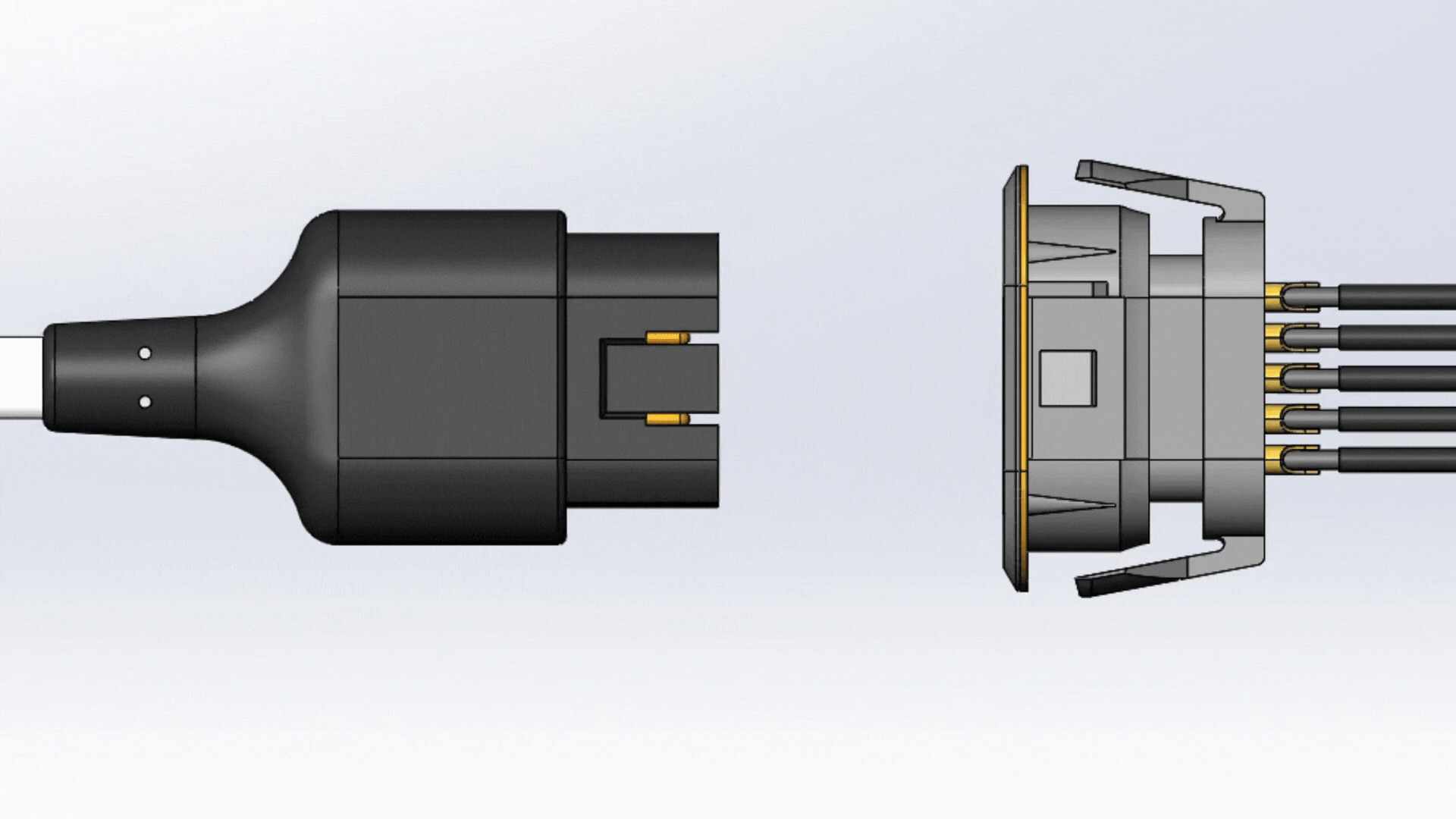
Front Shell
The front shell, as it is states, is placed in the front part of the connector. This is used to protect the pins and insulator from external debris and unnecessary movements.
Insulator
The insulator is wrapped around the contacts and solder cups. Insulation is used to minimize any unwanted interference that could cause any potential damages to the contacts.
Contacts
Contacts are the pins and sockets that bridge the electrical connection between the plug and receptacle. They are one of the main components (besides the cable itself) that transmit data signals and power to the mating receptacle. The size and number of contacts can limit the amount of voltage that can be applied before an electrical breakdown occurs.
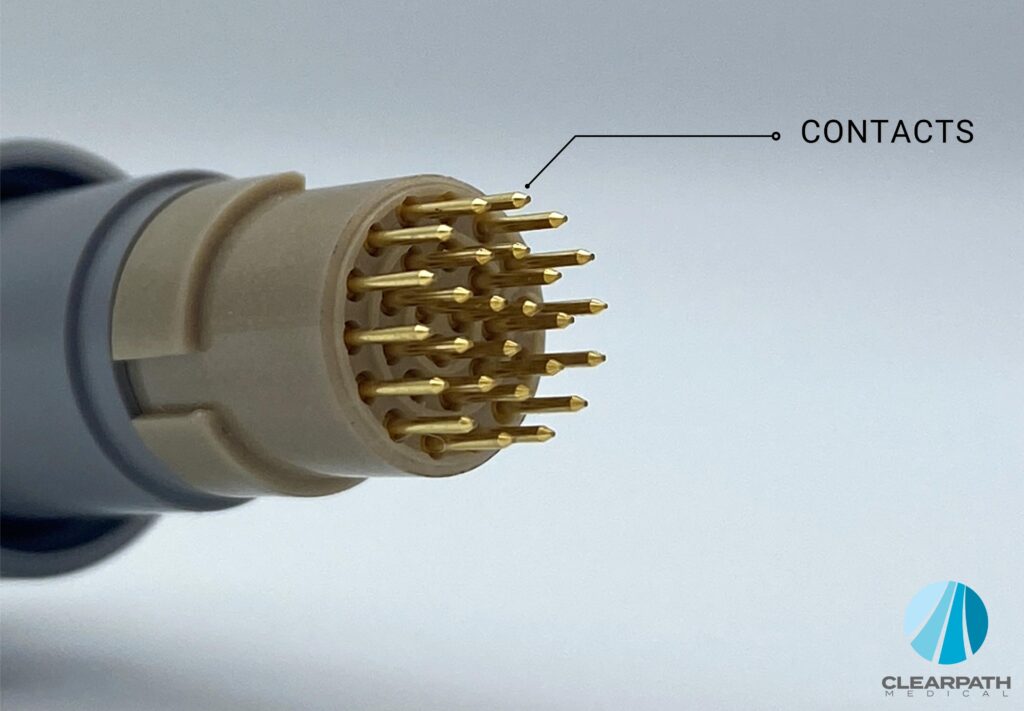
Some materials used for contacts in medical connectors are:
- Copper-based alloy
- Nickel plated with copper, gold or aluminum
Here’s a list of contact constructions:
- Stamped & Rolled Contacts – Open Barrel Pins and Sockets
- Machined Contacts – Closed Barrel Pins and Sockets
- Insulation Displacement Terminations
- Edge Card – Printed Circuit Board Interconnect

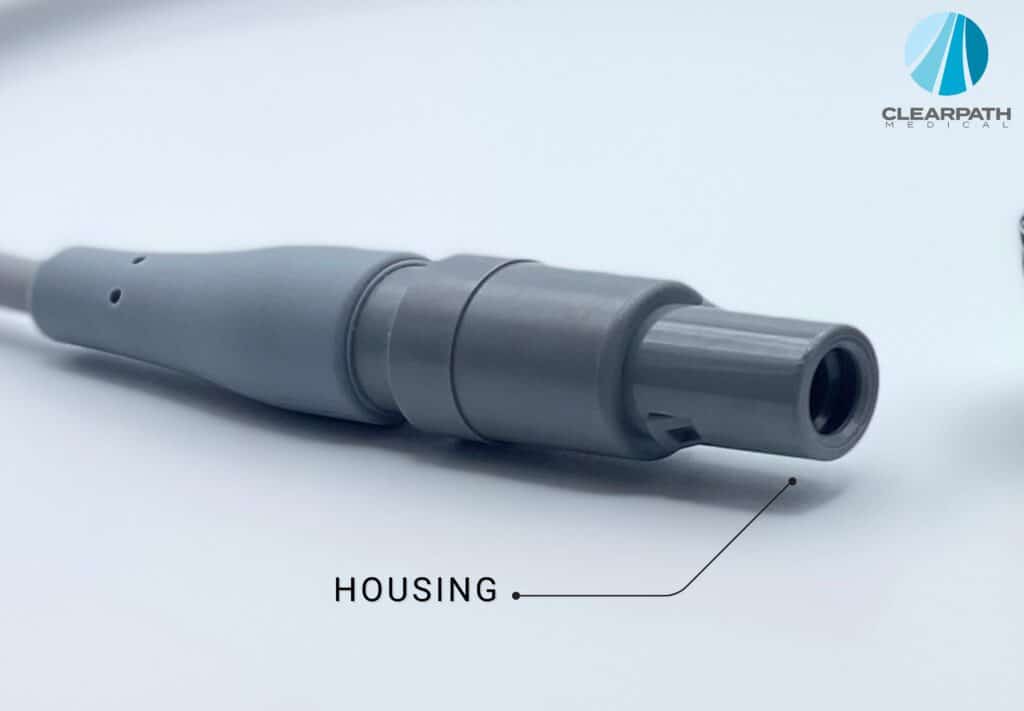
Housing
The final to the connector is its housing. The housing is used to make it easier to hold onto the connector and to insulate the contacts. The housing makes sure that the pins are protected from potential environmental damage, unwanted hazardous connections, and incorrect connecting orientation (keying).
Higher Standards = Trustworthy Connectors
In the medical device manufacturing industry, it’s crucial for medical devices to adhere to regulations and pass mate cycles. Having the proof to back up a manufacturer’s capabilities and quality ensures an accredited medical cable and connector.
Mating
One fundamental feature that guarantees a reliable connector is its ability to withstand a number of mate cycles. Mate cycles, or mate life, is defined as the number of times a connector can be connected and disconnected. Depending on how the connector is used, the connector should be able to properly mate and un-mate for up to tens of thousands of cycles. An example of this are patient monitoring devices. Patient monitoring devices are used daily, and the mate and un-mate cycle longevity are expected to last for several years.
Environmental Ramifications
Referring to the earlier portion of the blog, the purpose of housing is to protect the pins in a connector. These standards are made to help lower the risk of a bad product that could be life threatening.
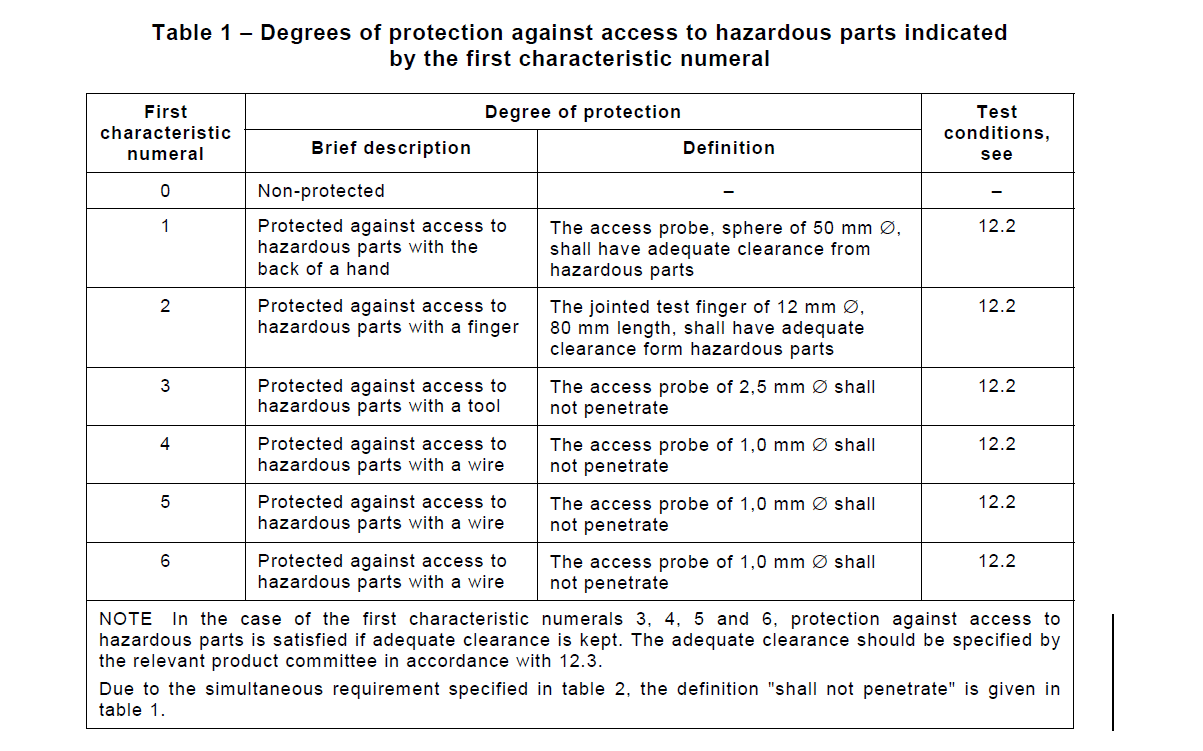
Ingress Protection
An example of environmental protection is the connector follows Ingress Protection or “IP” Code. To simplify the degrees of protection needed for connectors, one procedure that is commonly followed is through the International Electrotechnical Commission (IEC) Standard 60529.
An ingress protected connector means that it’s protected against ingress of water or moisture.
Water or moisture can cause electrical shorts between the connector’s contacts. This can lead to detrimental component damage, equipment damage, or environmental hazards (FIRE). It is important to identify IP rating requirements for connectors in both the mated and unmated condition.
Learn more about Ingress Protection & Medical Cables.
IEC 60601
For the safety of electronic equipment, IEC 60601 is a sequence of technical standards for the safety and credibility of medical electrical equipment. In addition, IEC 60601-1 is part of an FDA requirement for new devices. This standard is crucial when choosing a connector.
For more information, read more about FDA standards of IEC 60601
FDA Regulated
Other standards of regulation would be the U.S. Food and Drug Administration (FDA), where they regulate medical devices into three classes:
- Class I General Controls (lowest risk)
- Class II General Controls and Special Controls (higher risk)
- Class III General Controls and Premarket Approval (highest risk)
Determining the medical device’s risk class can lead to choosing the most appropriate medical connector for applications.
For more information, check out ISO and FDA Regulations.
What to Consider When Selecting a Connector for a Medical Cable Assembly
There are a lot of important decisions to make when selecting the most suitable connector for a medical cable assembly.
Here’s a simple checklist of things to consider:
- Will it be reusable or disposable?
- Locking or non-locking?
- Ingress Protected?
- Number of contacts? Low or High voltage?
- Unique keying?
- Commercial interconnect compatibility? (USB, HDMI, D-sub etc.)
- Ability to embed electronics (I.e. EEPROM)
Connecting It All Together
There are several components that go into a connector. Whether it’s the material, type of connector, usability, or protection, it’s important to understand what the process goes into it. ClearPath Medical will work with any type of connector and we are experienced in developing custom interconnects for your application. Looking to get started? Contact us for more information to get started on your next medical cable assembly.
Thanks for reading! Here are some more ClearPath Medical blogs that might interest you.
Love what you see? Sign up for our newsletter to get the latest updates on our company, worldwide and more.

